Introduction
This study investigates the relationship between cone photoreceptor loss, retinal microvasculature, and retinal sensitivity (RS) in non-pathological high myopia (HM). Traditionally, HM-related vision impairment has been attributed to biomechanical stress from axial elongation. However, this research highlights the independent role of deep capillary plexus (DCP) hypoperfusion in cone degeneration, challenging the purely biomechanical model. Using multimodal imaging, the study provides new insights into HM pathogenesis and potential biomarkers for early detection.
Method
The study employed a prospective, comparative design involving 43 non-pathological high myopia (HM) patients (spherical equivalent ≤ -6D or axial length ≥ 26mm) and 38 age-matched healthy controls. All participants underwent comprehensive ophthalmic evaluations including best-corrected visual acuity, axial length measurement, and multimodal imaging: adaptive optics scanning laser ophthalmoscopy (AO-SLO) for cone photoreceptor morphology assessment (density, spacing, dispersion, regularity at 3° eccentricity), spectral-domain OCT for retinal layer thickness measurements (MEZ, outer segments, central macula, choroid), OCT angiography for superficial and deep capillary plexus vessel density quantification, and microperimetry for retinal sensitivity assessment. Image analysis utilized automated segmentation with manual correction when needed, and all measurements were adjusted for axial length magnification effects using Bennett's formula. Statistical analyses included independent t-tests, ANCOVA, and multivariate regression to evaluate group differences and parameter correlations while controlling for age, with significance set at p<0.05. The study demonstrated excellent measurement repeatability (ICC 0.932-0.994 across all modalities).
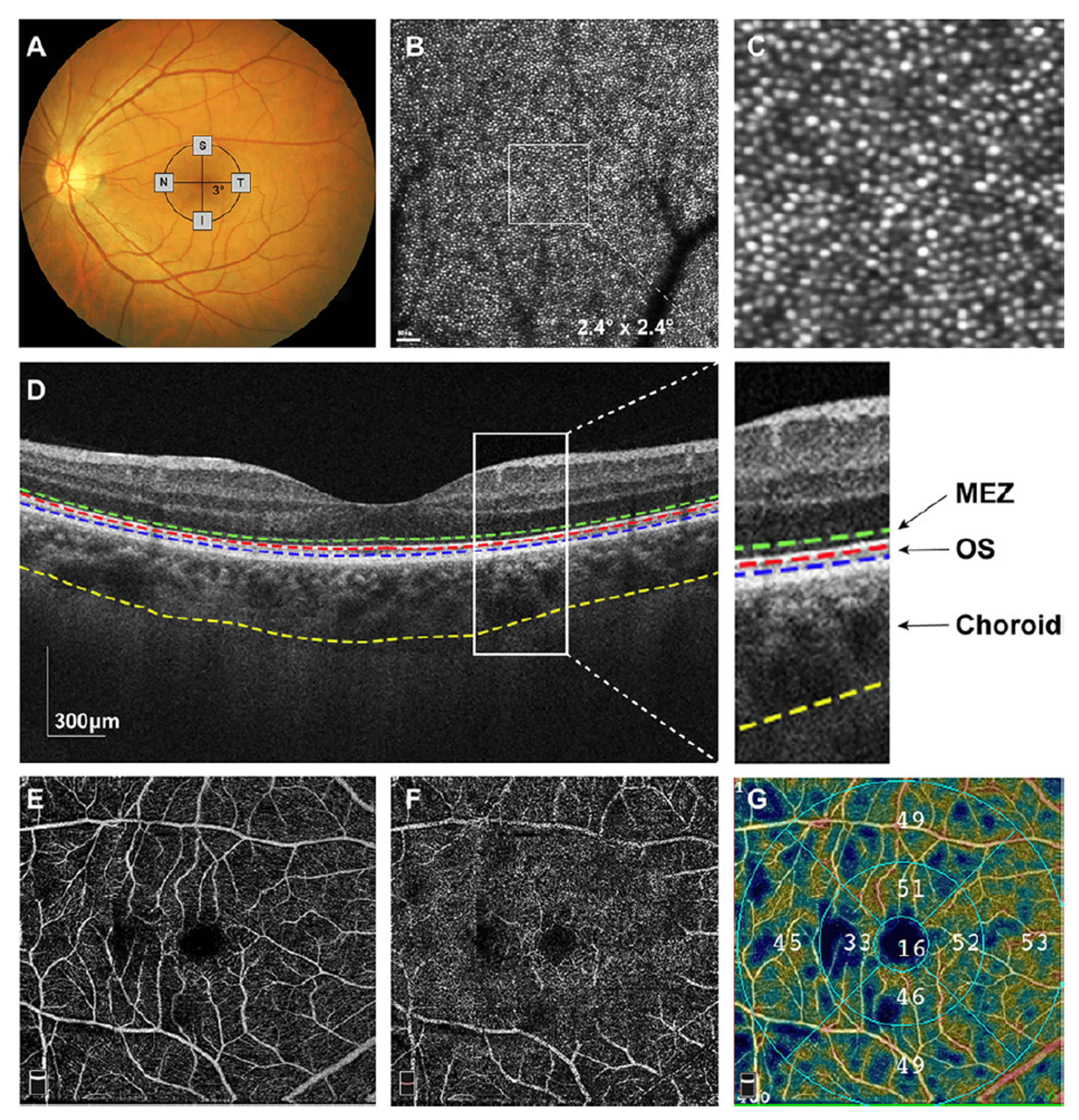
Results
The study revealed significant structural and functional alterations in high myopia (HM) through multimodal imaging analysis. AO-SLO imaging demonstrated markedly reduced cone photoreceptor density (15-22% lower across all quadrants, p<0.001) along with increased spacing, dispersion, and decreased regularity compared to controls. These cone abnormalities correlated strongly with impaired retinal sensitivity (r=0.689, p<0.001).

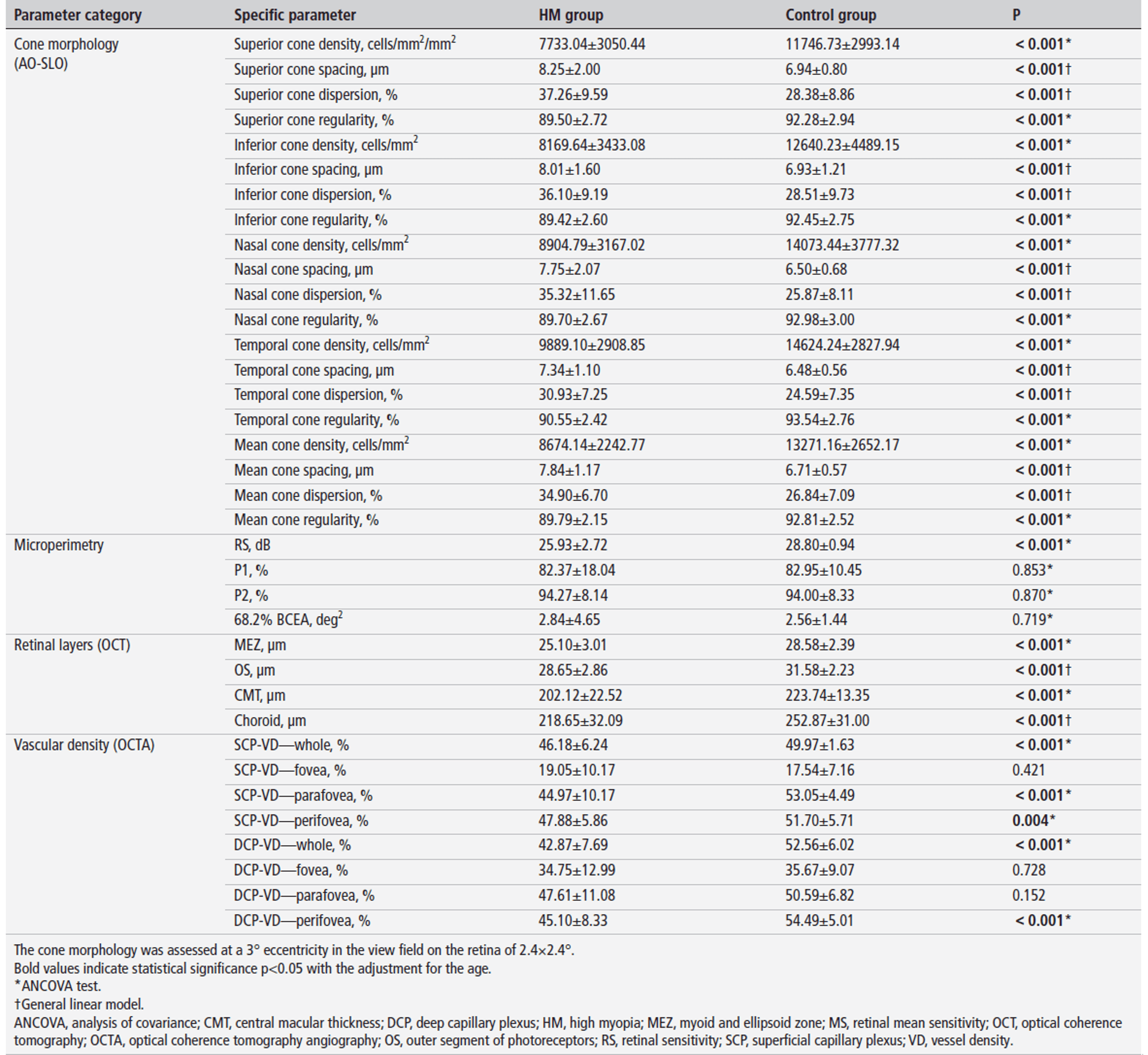
OCT measurements showed significant thinning of the MEZ layer (25.10±3.01 vs 28.58±2.39 μm, p<0.001), photoreceptor outer segments, central macula, and choroid in HM eyes.
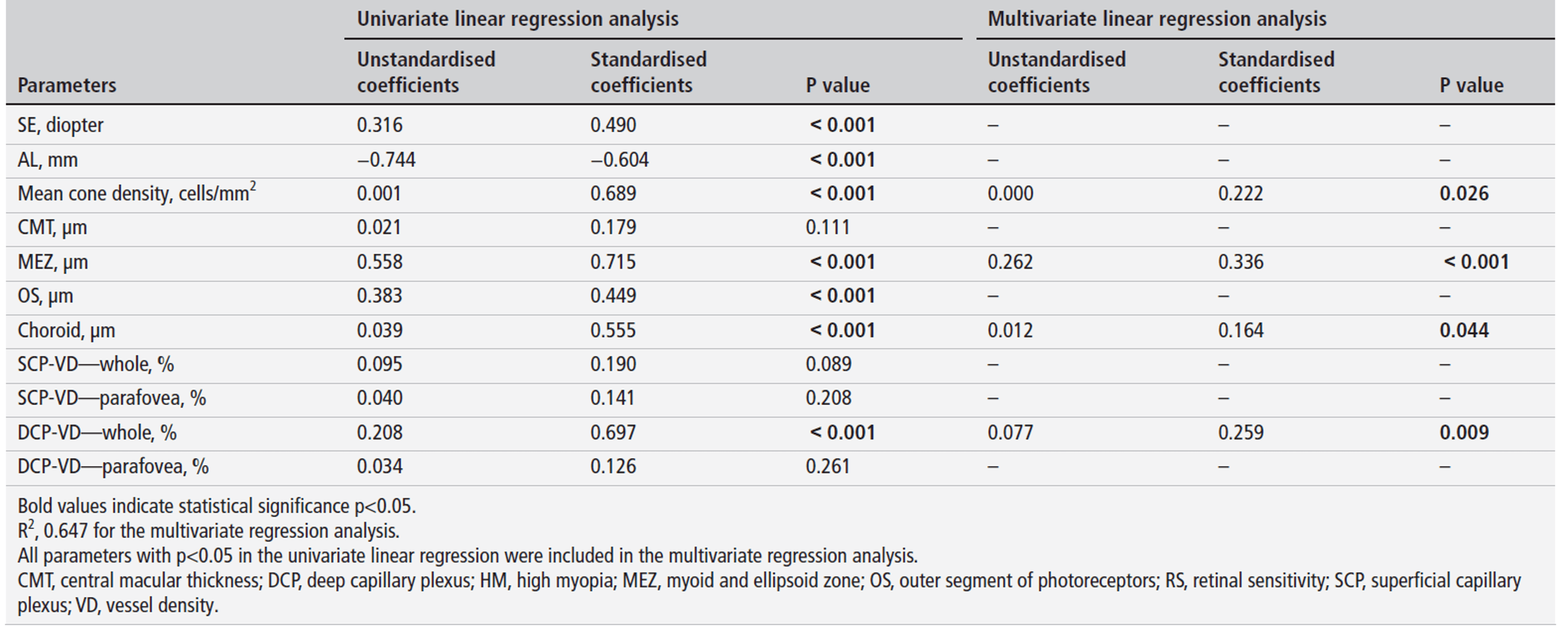
OCTA revealed reduced vessel density in both superficial and deep capillary plexuses, particularly in perifoveal regions (DCP: 45.10±8.33 vs 54.49±5.01%, p<0.001). Multivariate analysis identified axial length (β=-0.368, p=0.013) and deep capillary plexus vessel density (β=0.243, p=0.009) as independent predictors of cone density, while retinal sensitivity impairment was associated with cone loss, MEZ thinning, choroidal thinning, and DCP hypoperfusion (all p<0.05). These findings suggest HM involves synergistic microvascular and structural damage beyond simple mechanical stretching from axial elongation.
Conclusion
This study reveals that high myopia damages vision through both structural thinning (MEZ, choroid) and microvascular impairment (DCP hypoperfusion), with cone photoreceptor loss being independently linked to axial elongation and poor blood flow. These findings highlight the dual mechanical-vascular nature of myopic retinal degeneration, suggesting AO-SLO and OCTA biomarkers could improve early detection and monitoring.
Article Link: https://bjo.bmj.com/content/early/2025/06/17/bjo-2024-326609.long
